Institutional Review Board (IRB) Application Process at Management & Marketing, College of Business, Kean University (Wenzhou)
I would like to share this information about "Institutional Review Board (IRB) Application Process at Management & Marketing Department at College of Business and Public Management (CBPM) at Kean University (Wenzhou campus).
Different from another major, We, management and marketing faculty members and students, conduct field research to mine data from people. In this case, we need to comply "Human Subject Protection" rule because Kean University (Wenzhou) is subjected to the American education policy.
In order to apply and get an IRB approval, You can take these processes.
1. Please read and understand the IRB policy of Kean University
Kean IRB & Research Compliance
https://www.kean.edu/offices/research-and-sponsored-programs/irb-research-compliance/irb-applications-forms
IRB Applications & Forms | Kean University
www.kean.edu

Categories for Social Science and Educational Research could be equivocall to Exempt level or Expedited Level because many socience researches do not expriment on human subject directly. However, please consult it with IRB office if you conduct "Psychological or Physical Experiment" onto Human subject.
Necessary Document for Exempt or Expedited Level:

You must click according to the level of IRB category to submit your application.
Here are Exempt Application Form:
Form (in PPT)
Shortly speaking, you need to prepare "Required IRB Application Documents"
*Signed Paper Copy of the application (PI, CO PI and Faculty Advisor, if a student is the PI)
*CITI Certificate (PI, CO-PI and Faculty Advisor)
*Consent Form
*Debriefing Form
If applicable, these items must also be submitted with the application for it to be complete:
Assent Form (For participants under 18)
Site Permission
*Copies of all survey instruments, including interview questions
*Copies of recruitment letters, emails, flyers or advertisements
908-737-3461
Townsend Hall - 130 1000 Morris Ave Union, NJ 07083USA
2. You need to take an online course and you must get a Certificate of Tutorial Completion at here.
Overview of Training:
http://www.kean.edu/offices/research-and-sponsored-programs/irb-research-compliance/submission-information/training
CITI Training: https://about.citiprogram.org/en/homepage/
WKU IRB applicants are required to completing the Human Subject Research HSR) Series: Social, Behavioral, Educational Module.
Even though you already have a certificate from another institution, you are required to complete and show the certificate again for Kean University. It takes around 4~5 hours to complete the tutorial. You may prepare it in your free time before applying IRB.
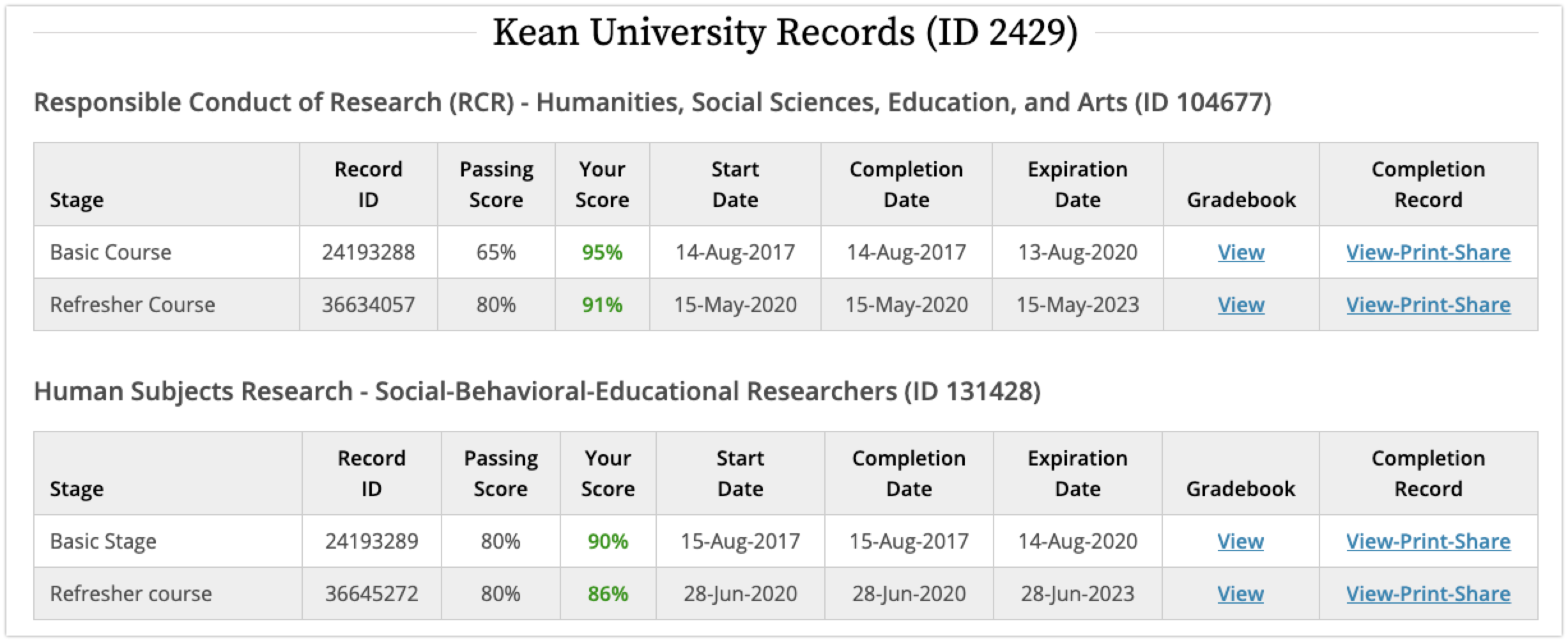
If you pass the Training Sessions, you will get two certificates:
1. RCR (Responsible Conduct of Research) - Humanities, Social Sciences, Educations, and Arts.
2. HSR (Human Subject Research) - Social Behavioral Educational Researchers.


3. Prepare the IRB application form.
By using an official application form, you can complete your IRB application form.
This is the official webform to apply for Kean IRB according to the level IRB.
https://keanirb.wixsite.com/keanirb
kean irb
keanirb.wixsite.com
You can download the IRB application forms in here.
https://www.kean.edu/offices/research-and-sponsored-programs/irb-research-compliance/irb-applications-forms
IRB Applications & Forms | Kean University
www.kean.edu
The IRB application level is totally dependent on your "subject".
If your IRB level is "Exempt" with minimal risk, it is automatically defined as an expedited application. And it will be much easier and faster. :)
4. Apply for IRB to Kean ORSP (Office of Research & Sponsored Program).
Once you complete 1) IRB application form and 2) Online course certificate, you can apply your IRB at the WEbsite.
(No Email, No personal submission; But Online application only)
IF you have any issue, you can contact, IRB COORDINATOR: irb@kean.edu
5. Then, Be patient.
Unfortunately, WKU cannot control the IRB process at Union campus. WKU is trying to take over the authority of "Exemption" level of IRB, but it is not positive because IRB authority cannot be delegated outside of the US territory. Anyway, I personally made a basic "understanding" with the IRB Office at Kean last winter. So, WKU management faculty will get a special support from the office.
You can refer the IRB Application Deadlines at Kean Union in here.
https://keanirb.wixsite.com/keanirb
Commonly, the IRB process requires "2 ~ 3 months" in general when it is "Exempt" level study because the IRB board meeting opens monthly for the 'Exempt" level. However, if you apply for level 1 or higher, it takes 4~6 months for review. But the time can vary depends on your research subject.
=======================
These are the rough guideline for your IRB application in WKU CBPM.
Jeonghwan (Jerry) Choi, PhD, MBA, ME
IRB Coordinator at Management & Marketing, CBPM, Kean University (Wenzhou campus)
=======================
Mar. 24, 2021 WKU get the official approval of IRB establishment


WKU Ethics Committee (exerpted in May 10, 2023).
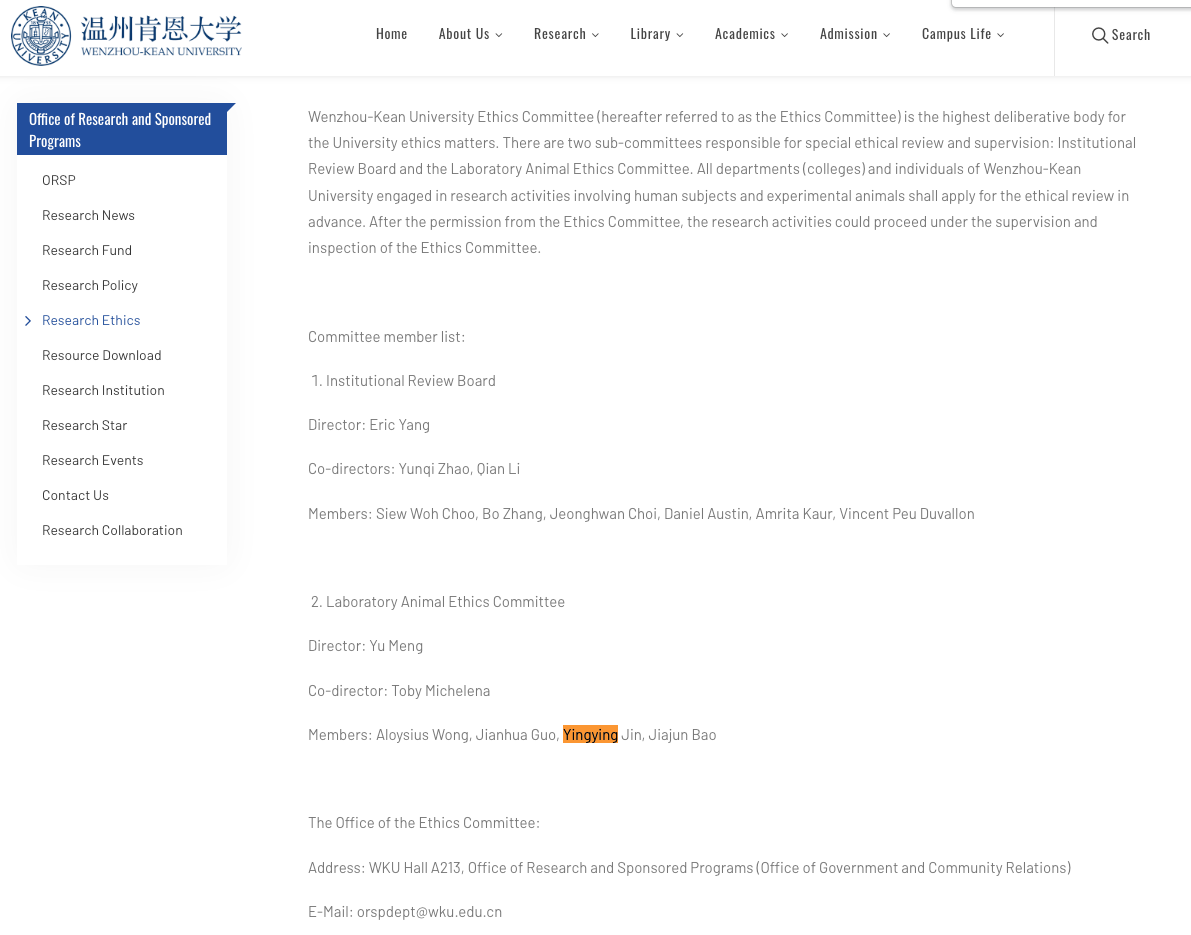
https://wku.edu.cn/en/org/orsp/research-ethics/
Research Ethics - Wenzhou-Kean University
Wenzhou-Kean University Ethics Committee (hereafter referred to as the Ethics Committee) is the highest deliberative body for the University ethics matters. There are two sub-committees responsible for special ethical review and supervision: Institutional
wku.edu.cn
2021. Mar. 24: Jin Chun (WKU ORSP) clarified the WKU needs to follow Kean USA IRB for research.
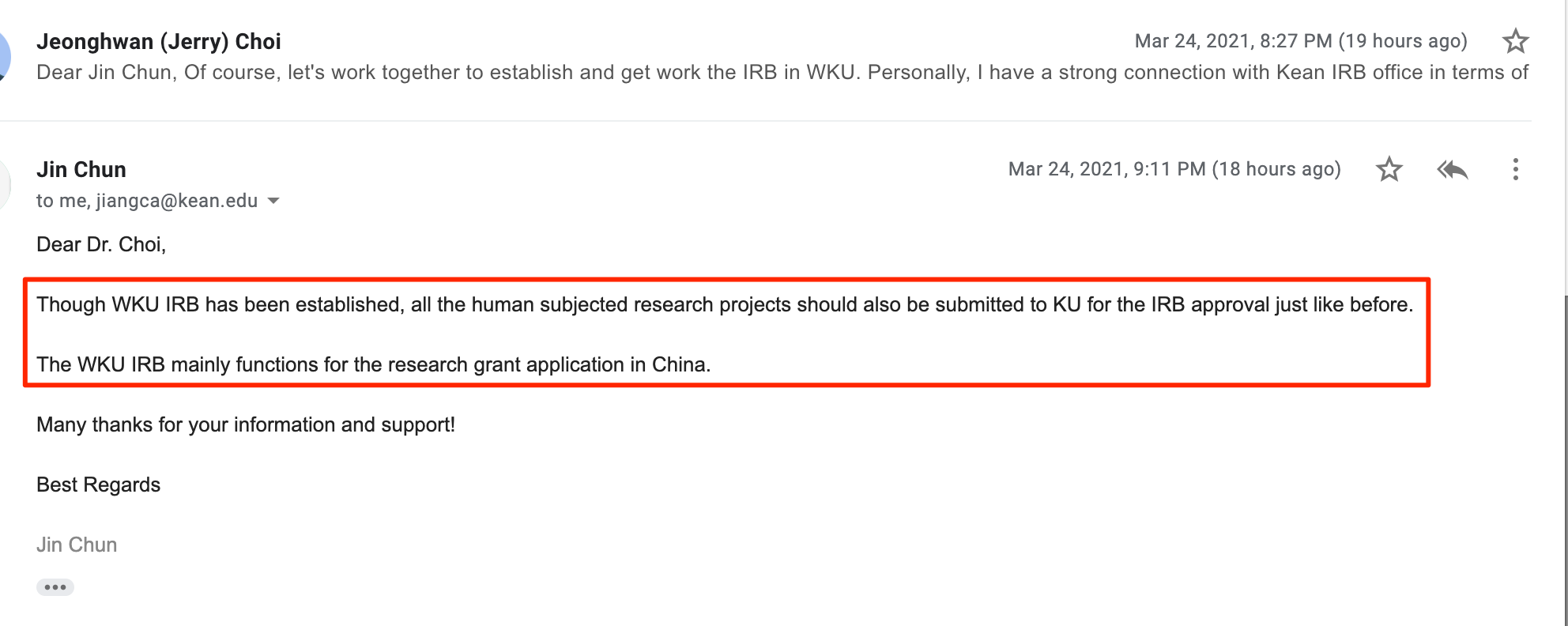
2021. 03. 25: Responding to the WKU IRB Issues and Clarification
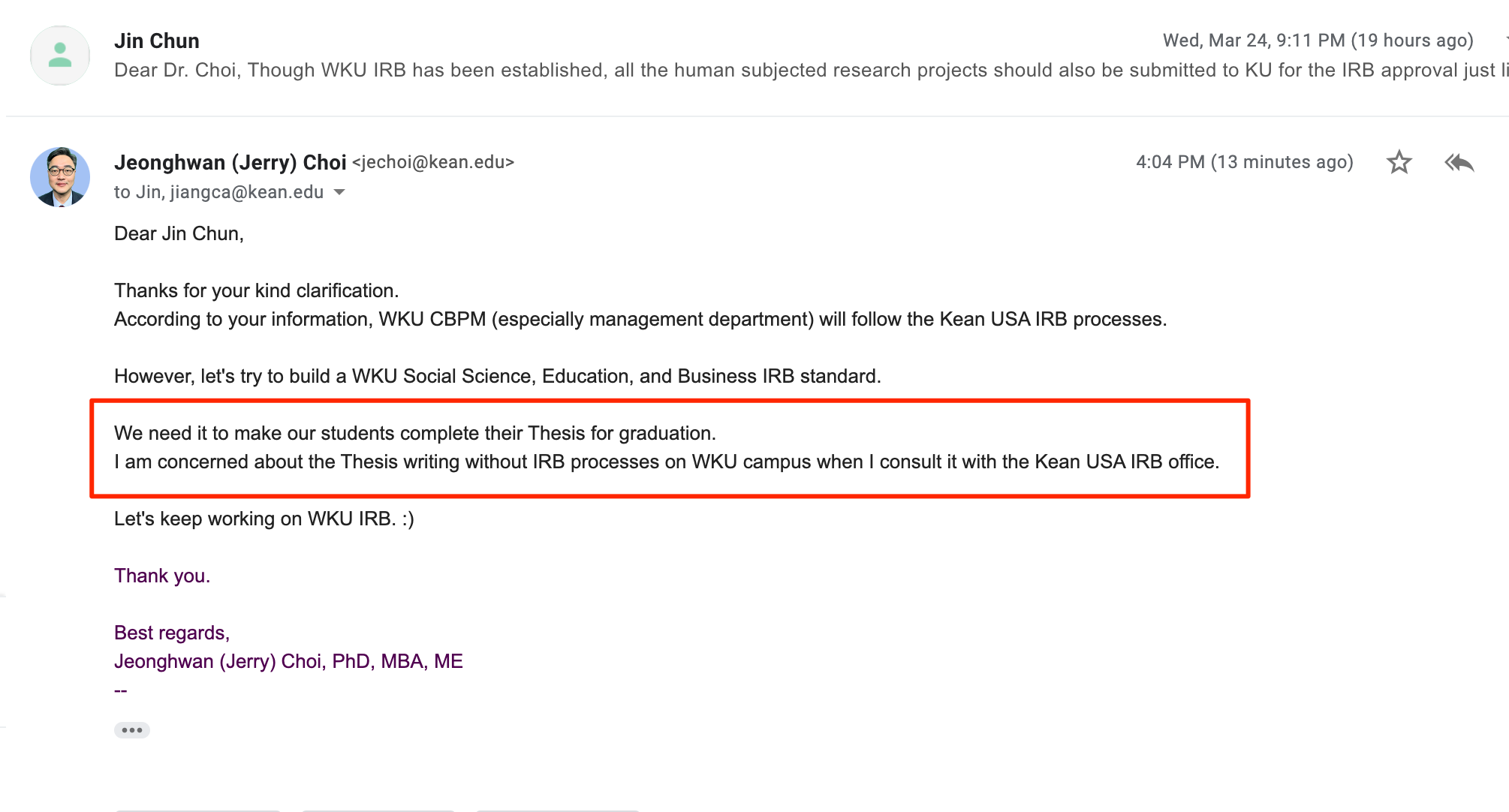

2021. Mar. 29: Suggesting The Hybrid Model of IRB for WKU

And I would like to suggest the "Hybrid Model of IRB for WKU" that probably overarching our unique situations.
The Hybrid Model of IRB for WKU
1. Qualification for Kean USA.
Kean USA has rich resources of online training and many practices for IRB application.
You can see several examples of mine here:
1) CITI Program (RCR, Humanitics, Social Science, Ecu cation, and Arts) Certificate.
2) CITI Program Social-Behavioral-Educational Researchers Certificate
https://leadershipcenter.tistory.com/451
If a WKU researcher (Principal Investigator) want to apply for the IRB, the researcher needs to complete Kean USA IRB qualification.
2. Chinese IRB application.
I consulted with the Kean USA IRB office several times. And the answer is solid and clear. Kean USA cannot establish an IRB office outside of the US Territories by Law. So, WKU needs to establish an IRB Office to apply, proceed, and protect research under the Chinese rule. Fortunately, IRB rules are universal regardless of borders. And, WKU - as a Chinese legal entity, we need to apply Chinese IRB.
So, I would like to suggest "Establishing the Hybrid Model of IRB for WKU that requests for the Kean USA level researcher qualification and Chinese IRB process".
This unique Hybrid model can help WKU and KU to show world class research and education!.
Let's keep working on it! :)
2021. Sept. 28. Ethics Committee Cover Social Science and Education as well (?)
(Contradictory to 2021. Mar. 25's comment - Social Science and Education IRB must go to Kean USA)

2021. Sept. 28: Social Science & Educational Research that is not for Chinese Research Fund must get Kean USA IRB.

2021. 10. 28: Regulation of WKU Ethics Committee, Clarification - NOT FOR "Social Science & Education" Research.
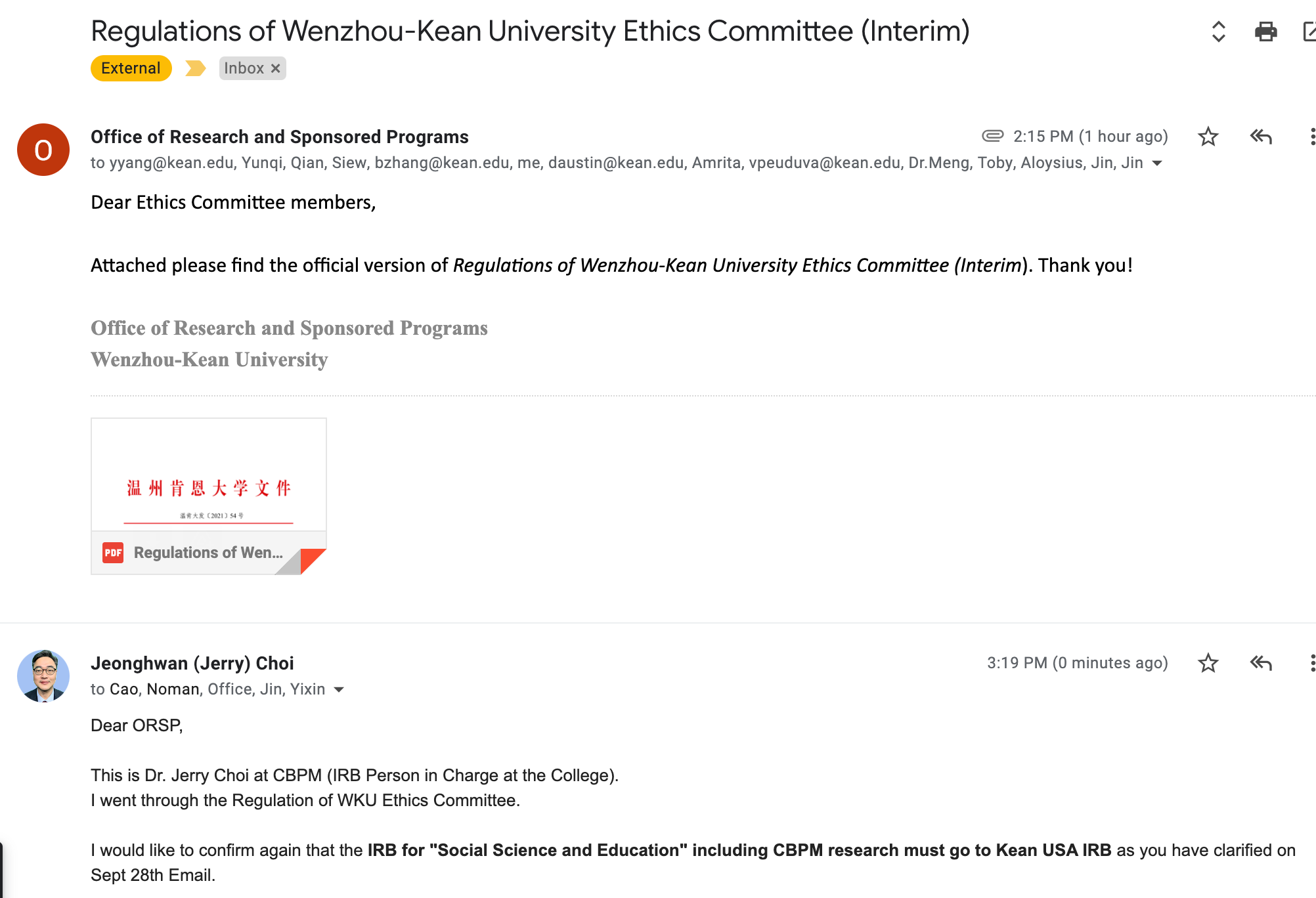
2022. 11. 14: Urgent request for "Completing CITI: IRB Members-Social-Behavioral-Educational Focus" Training course from WKU ORSP.
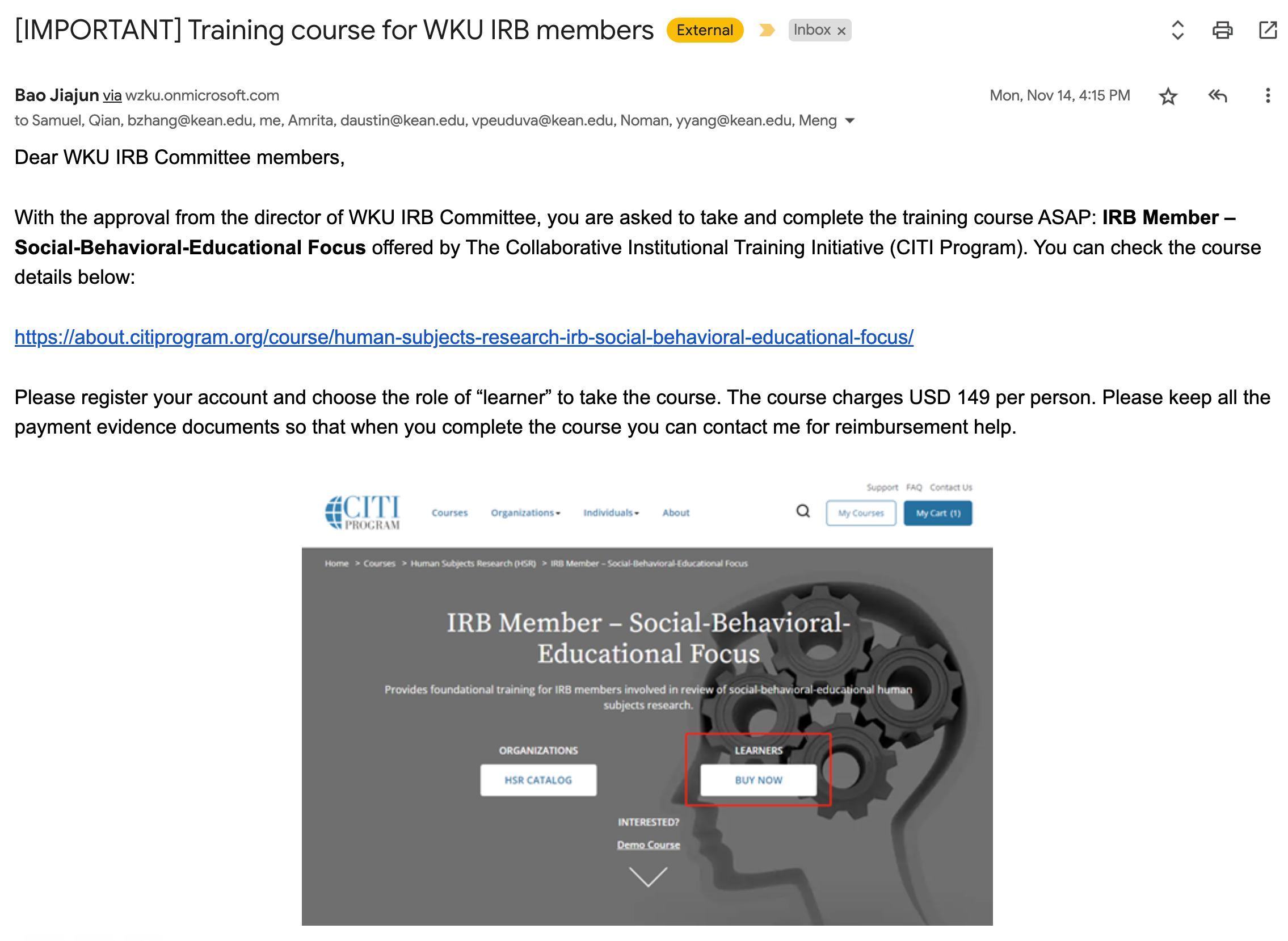
Follow-up communication confirmed that the course must be completed before end of Semester (Dec. 16, 2022) with personal expenses (Reimbursement is uncertain).
The training cost is $149 USD
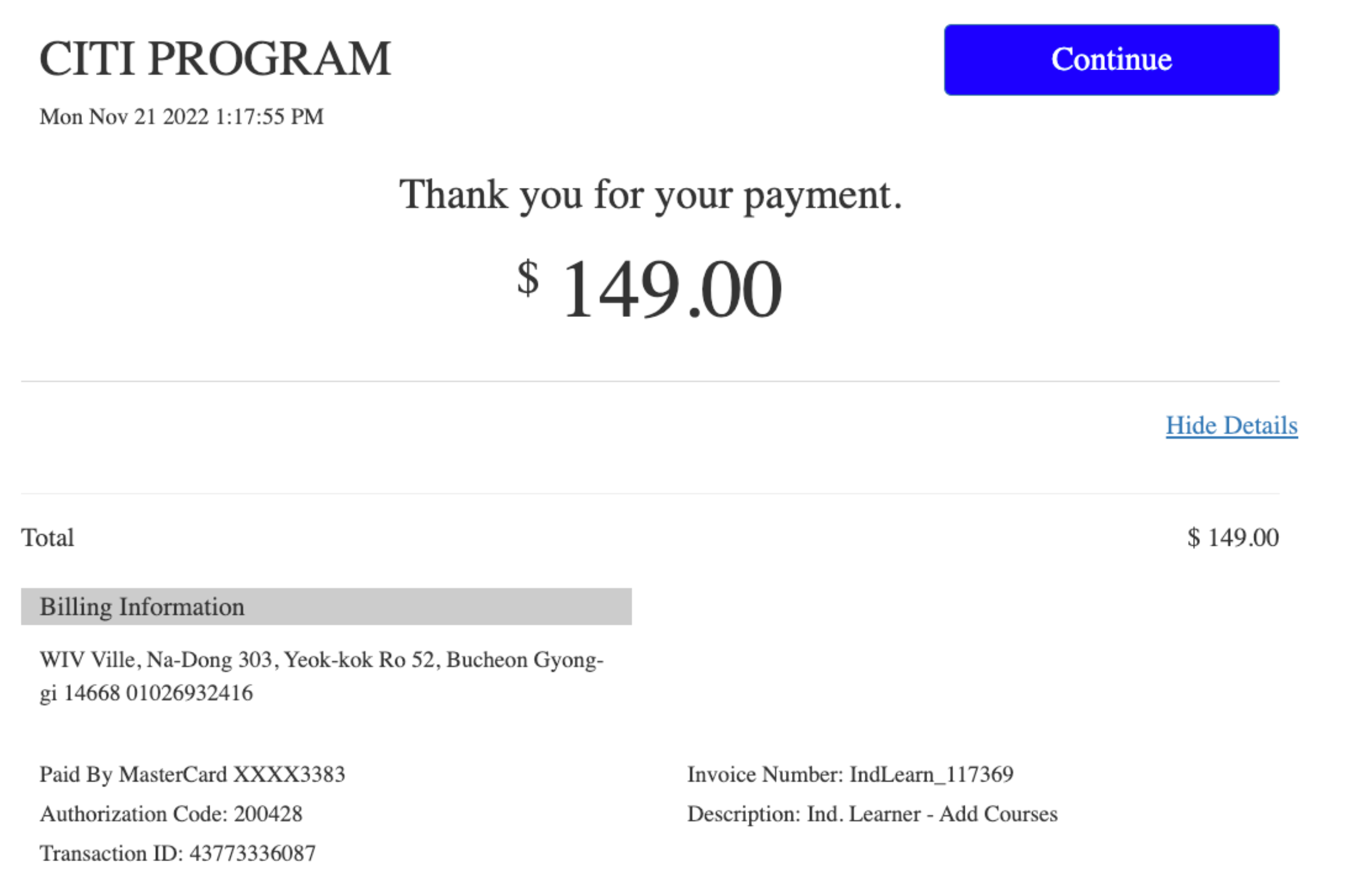
https://www.citiprogram.org/members/
CITI - Collaborative Institutional Training Initiative
Your session has expired. Please re-login. We use cookies and other tracking technologies to recognize your repeat visits and preferences, as well as to analyze traffic and measure the effectiveness of communications. To learn more, review our Cookie FAQ.
www.citiprogram.org
IRB Member Training Program has 17 Required modules. Approximately, every modeul needs around one hour committement to complete learninga and "Quiz".
Overall, Quiz must have at least 80% (4/5) accomplishment for each module. Overall, the score must be higher than 85%.
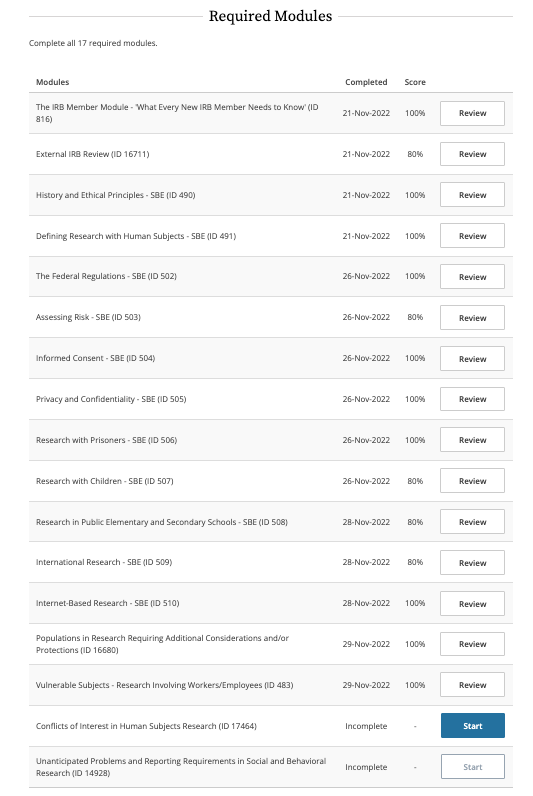
Completion of Training Course:
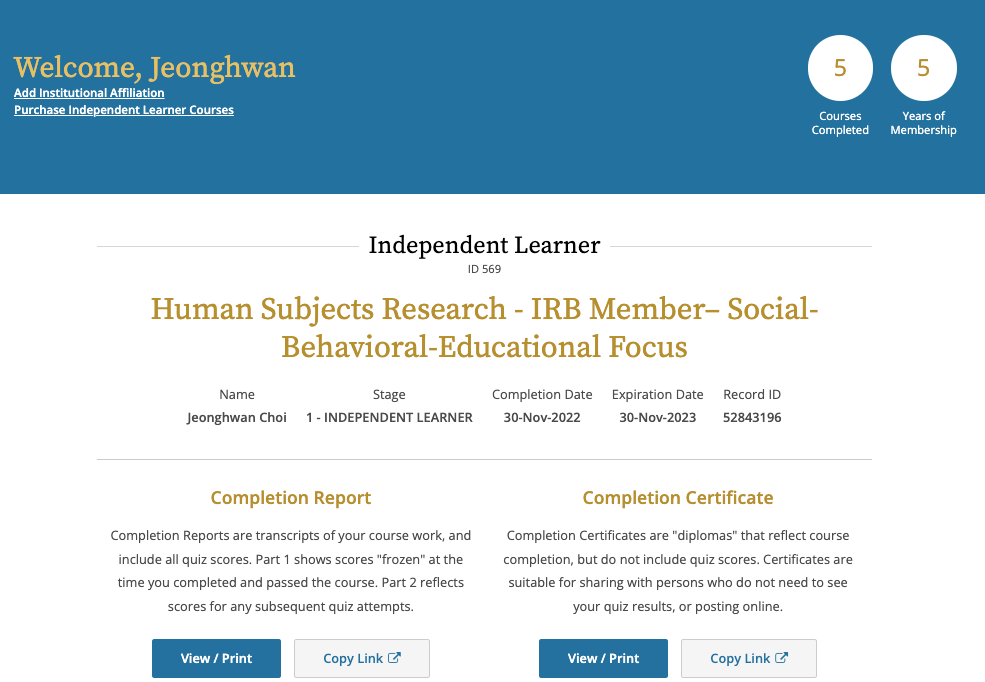

2023. 02.23: IRB applications Review could not be performed within WKU because WKU does not have a "Legitimated IRB Board" from Chinese Authorities. Either, there was no "Document" for IRB Board composition approval.
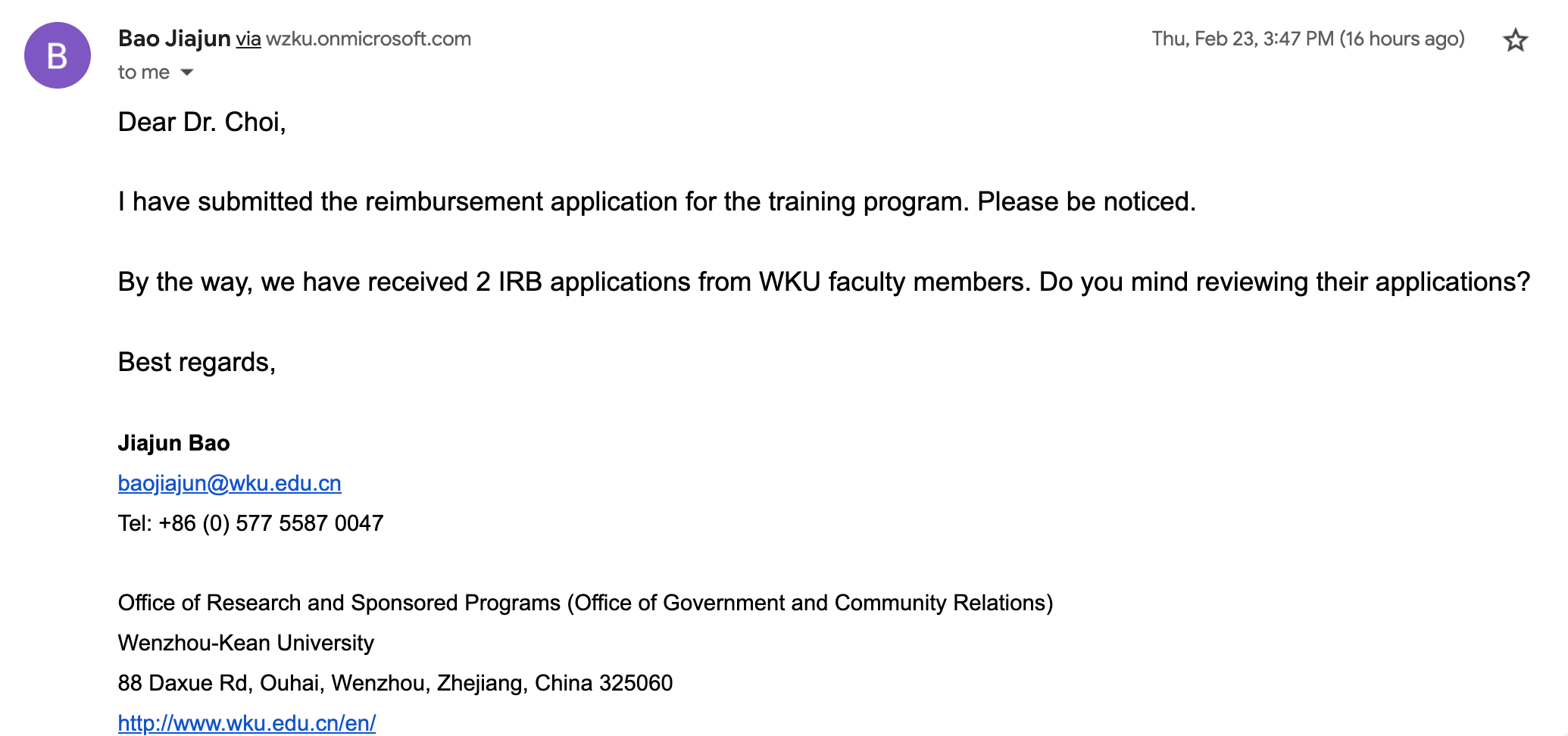

2023.04.01: Dr. Jerry Choi's Certificate of IRB Training Session: Human Subject Research: Social Behavioral Educational Research :

https://www.citiprogram.org/verify/?wb686a8cb-922c-40a0-9b04-92071f41d37b-55172347
CITI - Collaborative Institutional Training Initiative
Invalid verification code. Please contact the CITI Program Help Desk if you need assistance.
www.citiprogram.org
2023.04.03: Dr. Jerry Choi's Certificate of IRB Training Session: Human Subject Research: Humanities - Social Science - Education Research :
https://www.citiprogram.org/verify/?wa9edb058-94ba-419b-9175-b48ff7a07812-54413457
CITI - Collaborative Institutional Training Initiative
Invalid verification code. Please contact the CITI Program Help Desk if you need assistance.
www.citiprogram.org

2023. 04.07 (ORSP intentionally sent this request in late Friday to kill three working days for recipients): WKU ORSP sent an urgent review of four policy documents for WKU IRB's service with unclear scope of the purpose or sufficient information. The office request for the review by three workind days (April 12, 2023).
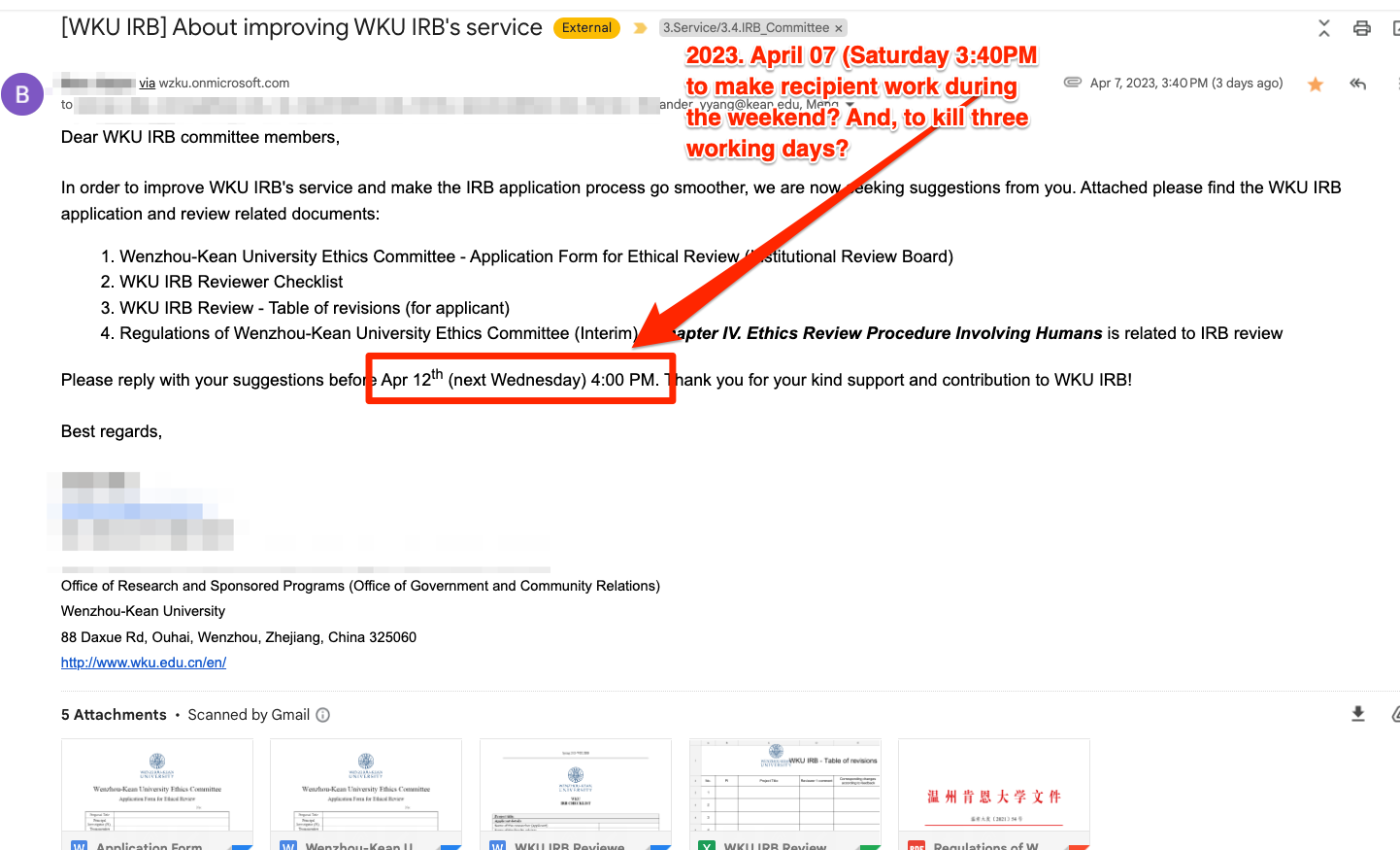
1. Application Form for Ethical Review (Institutional Review Board)_Apr 2023
2. Wenzhou-Kean University Ethics Committee - Application Form for Ethical Review (Institutional Review Board)_Apr 2023
3. WKU IRB Reviewer Checklist_Apr 2023
4. Regulations of Wenzhou-Kean University Ethics Committee (Interim)温州肯恩大学关于印发《温州肯恩大学伦理委员会章程(试行)》的通知
5. WKU IRB Review - Table of revisions (for applicant)
2023. 04.08 (Working on Saturday for ORSP intentionally sent this request in late Friday to kill three working days for recipients): Dr. Choi raised 7 concerns and suggestions that needs to be addressed before reviweing the WKU IRB Office Establishment.

Before reviewing the policy and procedure, I have several questions about establishing an IRB Office on the WKU campus.
1. From where does the WKU IRB Office obtain accreditation for legitimacy and IRB numbers? For example, Kean USA complies with the US Department of Health and Human Services (HHS) and the Association for the Accreditation of Human Research Protection Programs (AAHRPP) to gain authority and operate their IRB office. Please see the Kean USA IRB Office Website: https://www.kean.edu/offices/research-and-sponsored-programs/irb-research-compliance
2. Who is the designated IRB administrator? The WKU IRB Office needs a fully engaged and designated IRB Administrator with expertise and capability in managing the office.
In March 24, 2021, WKU ORSP announced this:
Announcement of Name Change and Membership Adjustment of WKU Ethics Committee of Life and Medical Science: Institutional_Review_Board__IRB__Application_Process_at_Management___Marketing__College_of_Business__Kean_University__Wenzhou_.png At that time, the IRB board and Lab Animal Ethics committee was formed for Natural Science audit with 'unclear' administrator designation.
For better procedural management, We need a clear "Person-in-Charge" of IRB for Natural Science, IRB for Social Science.
3. What are the boundaries of the IRB?
The description states, "Regulations for Ethical Review of Biomedical Research Involving Human Subjects, Regulations for the Management of Laboratory Animals, and relevant national laws, regulations, and international practices; these Regulations are hereby formulated to regulate Wenzhou-Kean University's conduct of relevant experiments involving human subjects and animals, respect and protect the legal rights and interests of human subjects, safeguard animal welfare, and standardize ethical review and professional behavior of practitioners."
- Ethical Review Boards and Institutional Review Boards are not the same and should have different boundaries.
- The description mentions "Natural Science" but does not imply "Social Science." The boundaries between natural science and social science should be clearly defined separately.
- The IRB review board for natural science and social science should be clearly defined independently.
4. For reporting, the checklist you suggested is only for "Social Science." A reporting format for natural science, especially for biomedical and animal experiments, is necessary.
5. Regular audits? In connection with question #1, which entity provides regular audits and accreditation renewal for the WKU IRB office? (A specific government authority should be identified).
6. What kind of education and qualifications are required and mandated for IRB applications and/or IRB approval? We can rely on Kean USA IRB education (Online) for certification, but I am not sure whether that is compatible with China's requirements.
7, We can consult the Kean USA - WKU IRB cooperation with ORSP, IRB Coordinator, irb@kean.edu. She can help us.

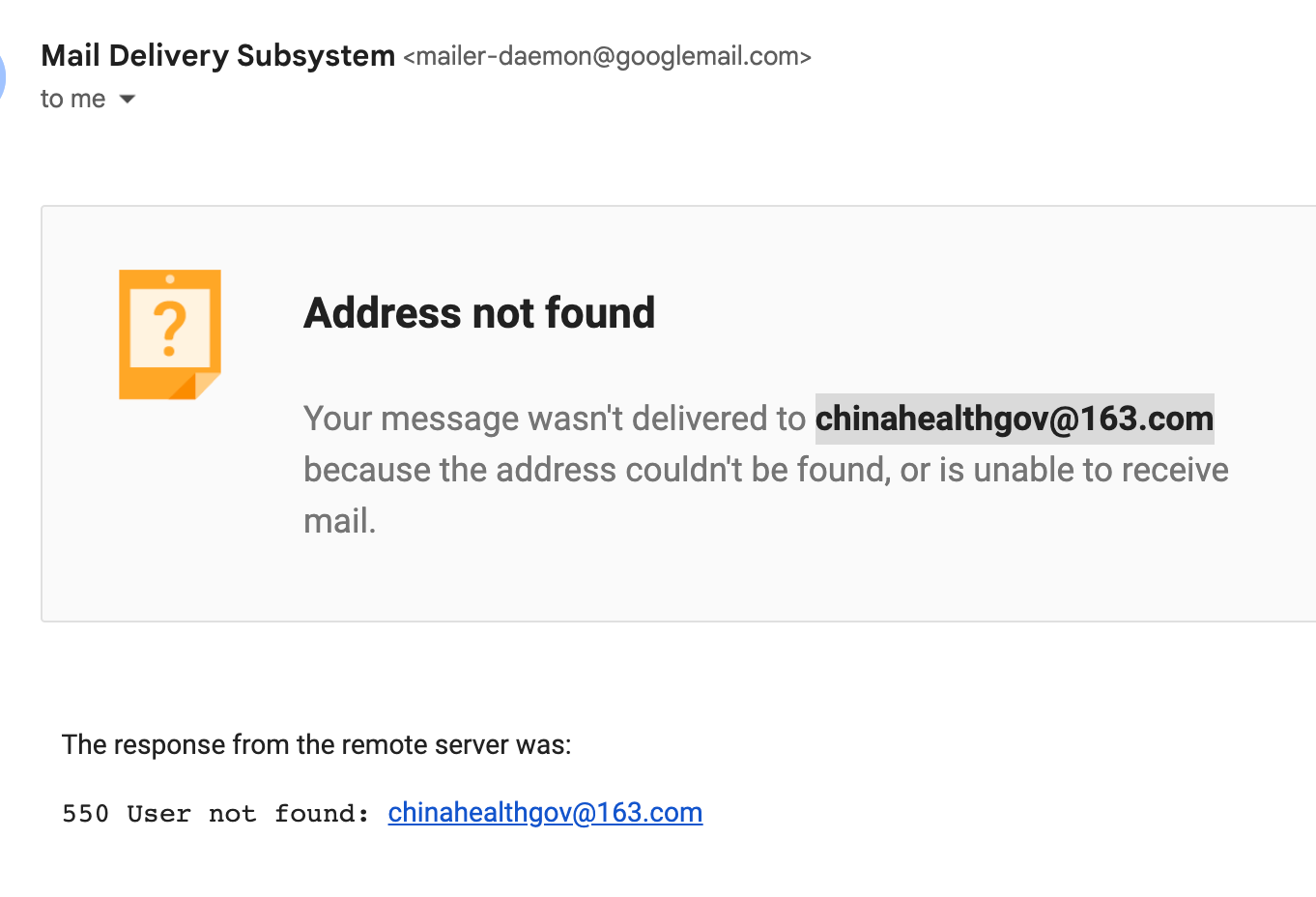
2023. 04.10 Checking the Chinese IRB Office Operation and Sent an Inquiry Email to the NHC (chinahealthgov@163.com) but failed.
In China, the National Health Commission (NHC) and its ethical review committees are responsible for overseeing research involving human subjects. While there isn't a direct equivalent to AAHRPP in China, the NHC and its ethical review committees set the standards and guidelines for conducting human subject research and establish regulations for the operation of IRB offices in China.
For institutions in China seeking to operate an IRB office, they should consult and adhere to the guidelines and regulations established by the NHC, as well as any additional local and provincial regulations or requirements.
To consult about IRB office operation in China with the National Health Commission (NHC), you can start by visiting their official website (http://en.nhc.gov.cn/) to gather information and find relevant contact details. You may also consider reaching out to your institution's research administration office, as they may have more information on the appropriate contacts within NHC for your specific needs.
National Health Commission of the PRC
What we do To draft laws and regulations for national health policies, draft laws and regulations, policies and plans for the development of public health services, formulate departmental rules and standards and implement them; coordinate and plan the reso
en.nhc.gov.cn
Keep in mind that information on the NHC website is primarily in Chinese. You may need to use translation tools or seek help from a Chinese-speaking colleague to navigate the site and find relevant contact information.
Additionally, as the NHC is a government organization, it's possible that there may be different departments or offices within the organization responsible for various aspects of research oversight. It might take some effort to identify the right office or department to consult about IRB office operations.

2023. 05. 29: IRB Issue Discussion among Faculty Members.
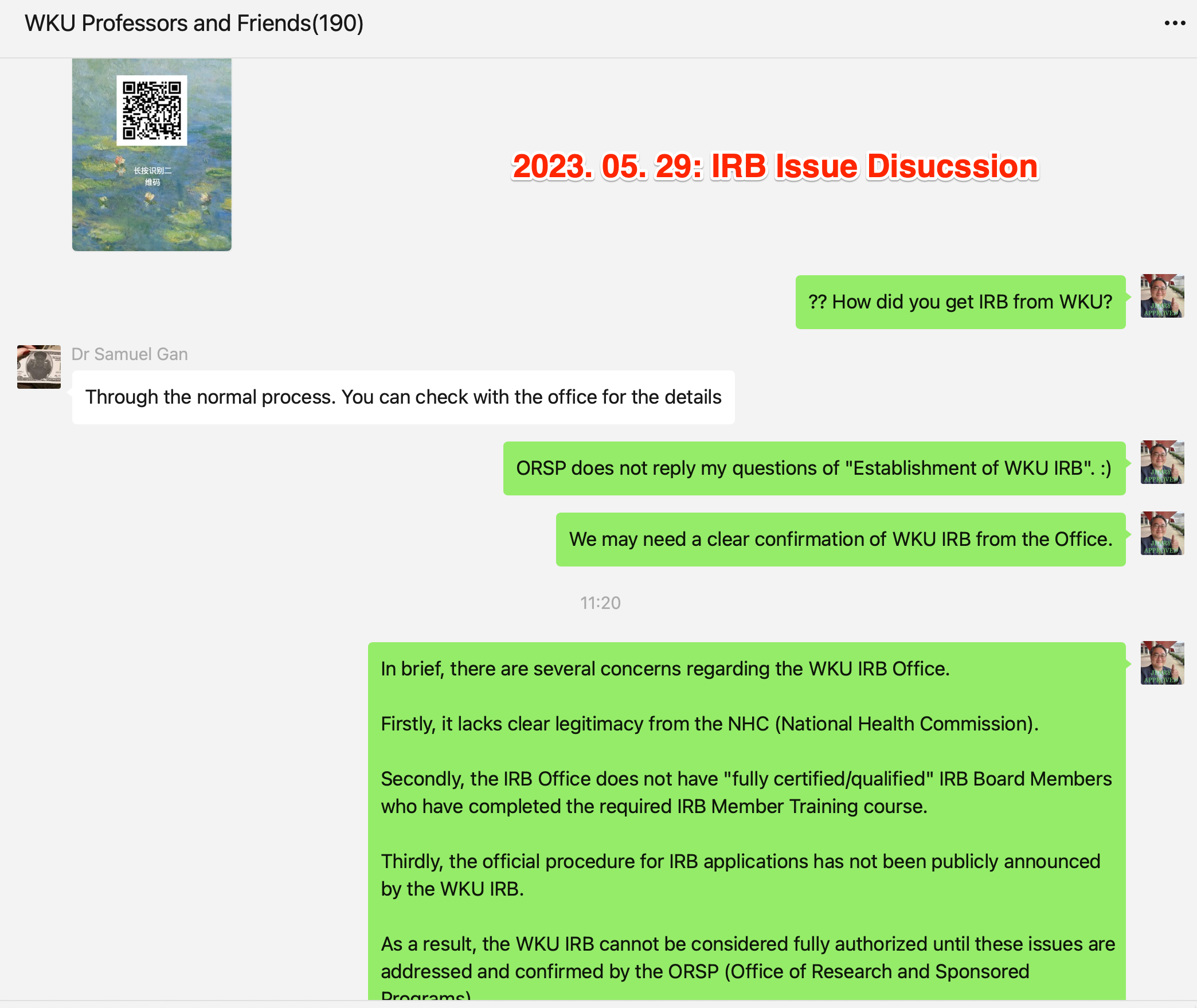
In brief, there are several concerns regarding the WKU IRB Office.
Firstly, it lacks clear legitimacy from the NHC (National Health Commission).
Secondly, the IRB Office does not have "fully certified/qualified" IRB Board Members who have completed the required IRB Member Training course.
Thirdly, the official procedure for IRB applications has not been publicly announced by the WKU IRB.
As a result, the WKU IRB cannot be considered fully authorized until these issues are addressed and confirmed by the ORSP (Office of Research and Sponsored Programs).
2023. 06. 01: Questions and Answer with Dr. David Birdsell (Senior Provost of Kean USA @ WKU Faculty PDD).
2023. 06 .01: Send an Email to Dr. Birdsell to update the IRB issue of Wenzhou campus.

2023. 07.03 : EXAMPLE OF CHINESE IRB - Ethics Approval from HREC (Human Research Ethics Committee) Approval Example of The University of Hong Kong

http://www.rss.hku.hk/integrity/ethics-compliance/hrec
Research Services - Human Research Ethics Committee (HREC)
Human Research Ethics Committee (HREC) In line with the HKU Policy on Research Integrity, Principal Investigators (PIs) who are academic/research staff members or research students (MPhil/PhD) in Faculties other than Medicine and Dentistry should apply to
www.rss.hku.hk
2023. 07.14 : A Chinese University in the mainland does not have nor operate the Ethics Committee.

2023.09.14. Kan USA IRB Office updated the Form of IRB application (Exempt or Expidited). All IRB for Kean IRB approval should use the new form.
Exempt Form:
Example of Exempt Form: Dr. Jerry Choi's Agile Study
2023. 11. 02. A Faculty Colleague share the Kean niversity (Wenzhou campus) newly lanuched IRB Procedure for SpF.
* According the official Website, there are IRB Board members including Dr. Jeonghwan Choi. But, the notification "WKU IRB" documentation and procedures were never officialized by Nov. 02, 2023.
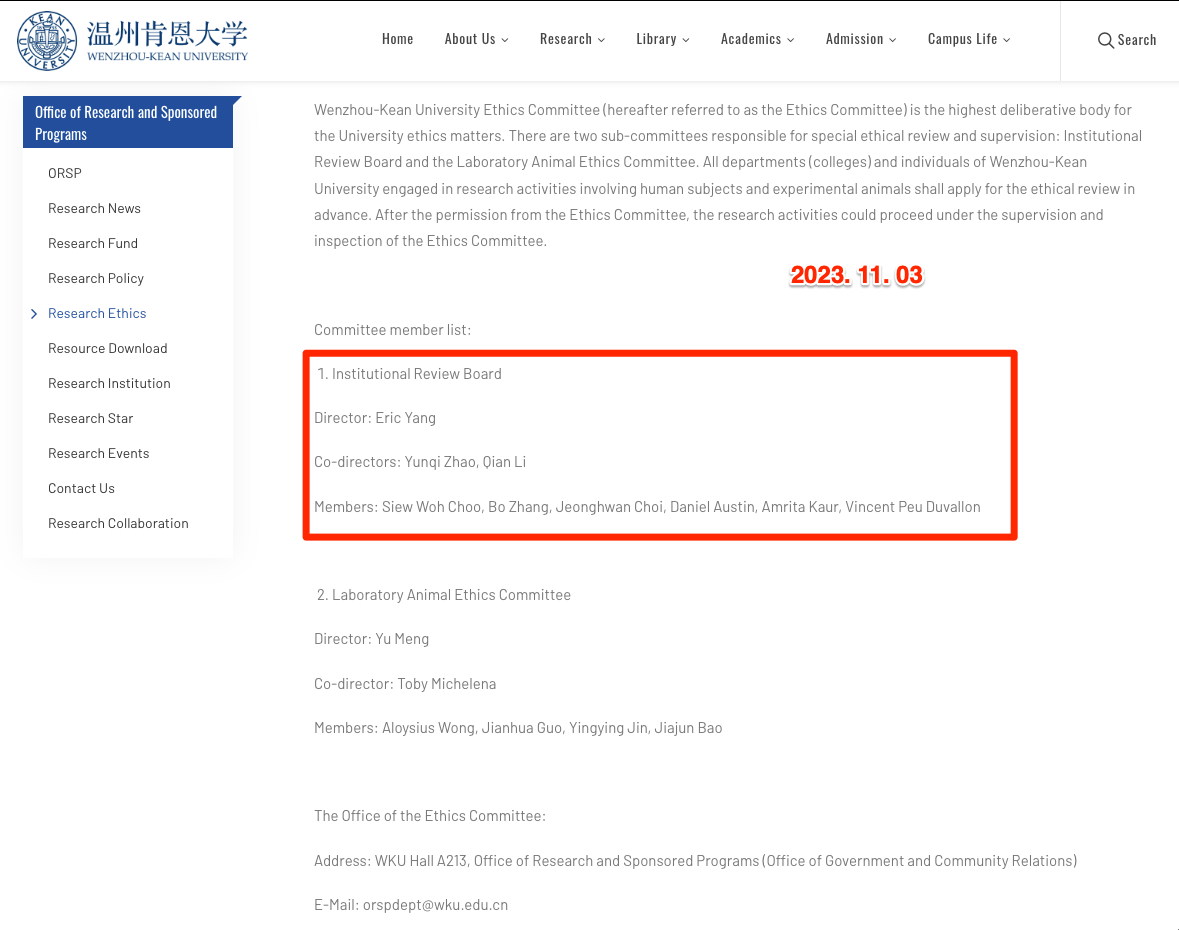
Here are WKU IRB Dcoumentaion Resources:
https://www.wku.edu.cn/en/org/orsp/resource-download/
Resource Download - Wenzhou-Kean University
www.wku.edu.cn
Although it is not publicly announced, it contains the documentation for IRB Process in the Wenzhou campus.
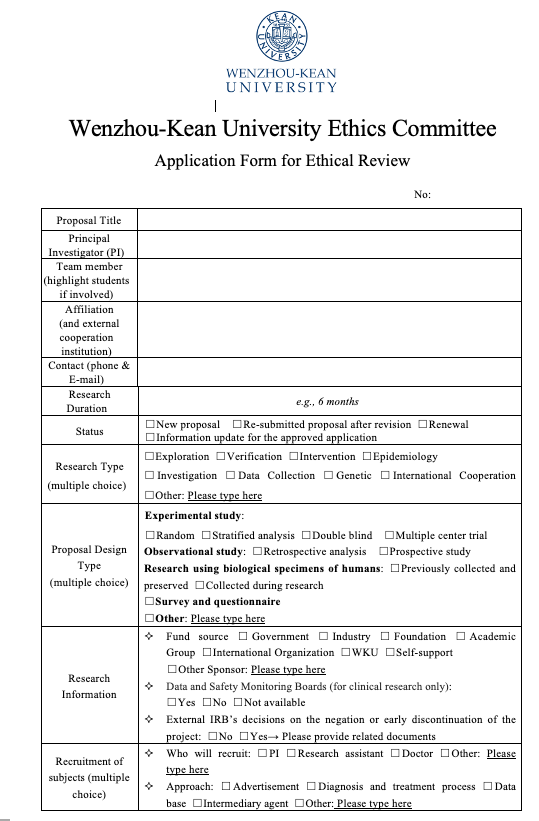
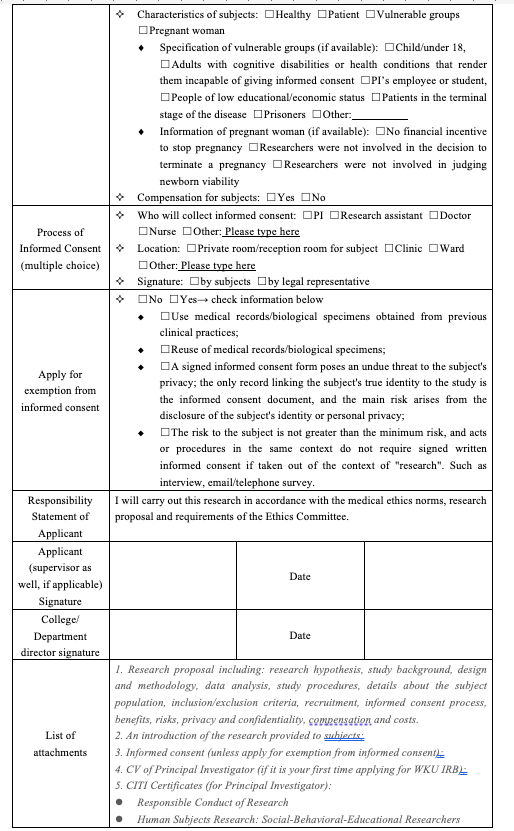

Self_Checklist:
2023. 12. 31: The IRB problem of WKU CBPM remained unresolved until the end of 2023.
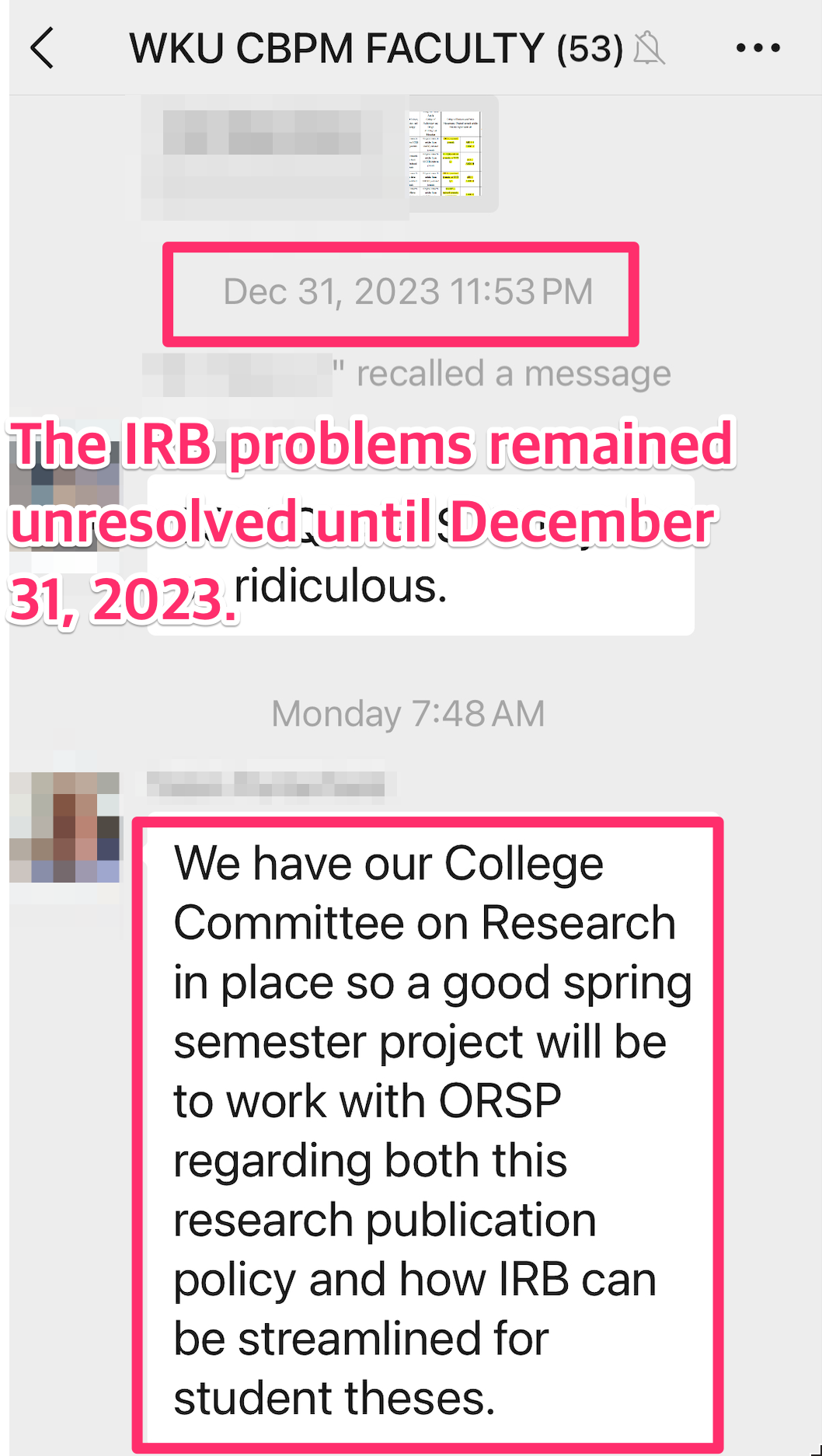
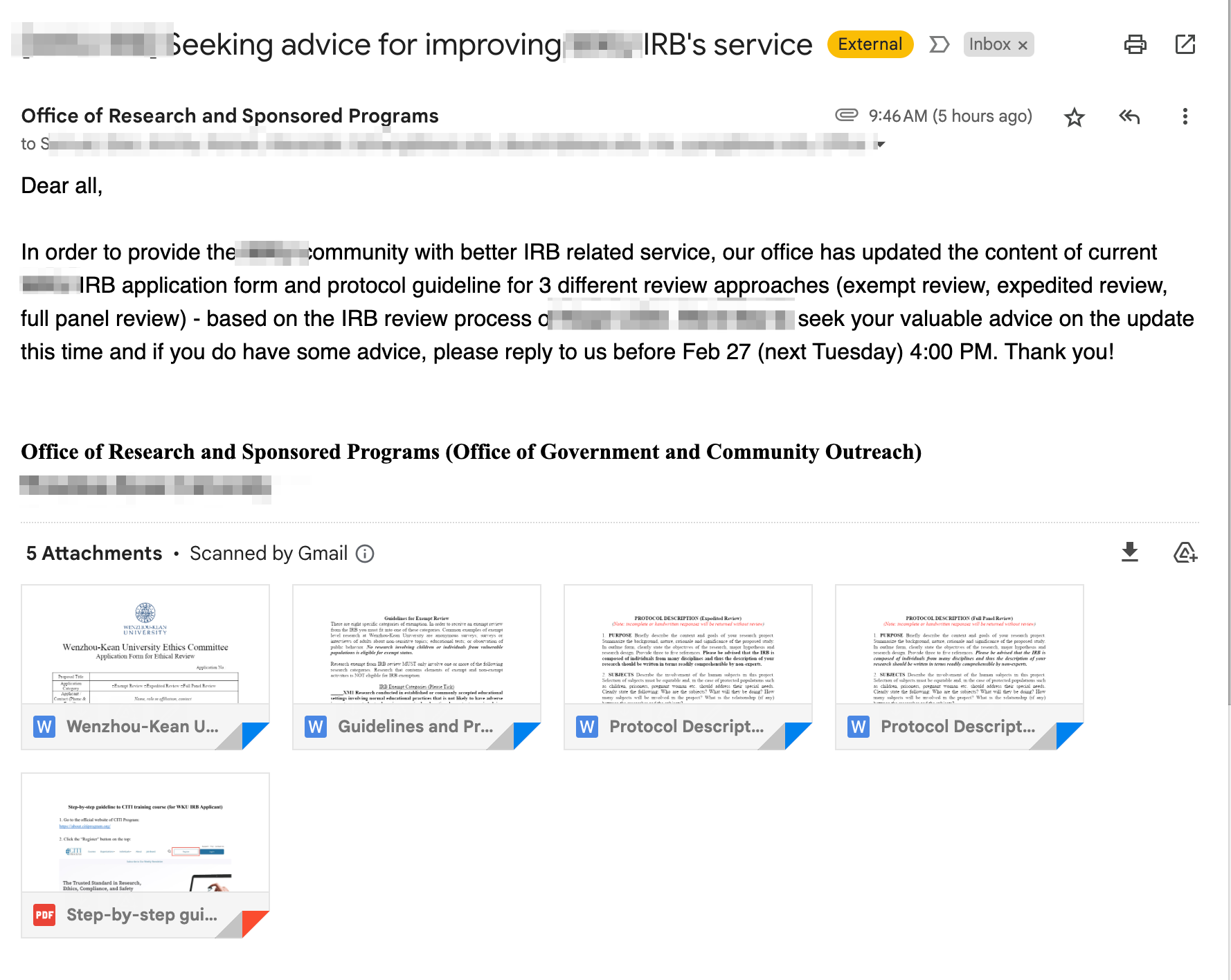
2024. 02 .23: Demand Improvement ofIRB
====================
2023. 12. 31: The IRB problem of WKU CBPM remained unresolved until the end of 2023.
2023. 11. 02. A Faculty Colleague share the Kean university (Wenzhou campus) newly lanuched IRB Procedure for SpF. But it was not officiall announced for Researchers.
2023. 09. 15 : New Forms for Kean IRB were updated at the Site. In addition, the procedure of IRB application was amended. All IRB application should be submitted via System.
IRB Applications & Forms | Kean University
www.kean.edu
2023. 07.14 : A Chinese University in the mainland does not have nor operate the Ethics Committee.
2023. 07.03 : EXAMPLE OF CHINESE IRB - Ethics Approval from HREC (Human Research Ethics Committee) Approval Example of The University of Hong Kong
2023. 06 .01: Send an Email to Dr. Birdsell to update the IRB issue of Wenzhou campus.
2023. 06. 01: Questions and Answer with Dr. David Birdsell (Senior Provost of Kean USA @ WKU Faculty PDD).
2023. 05. 29: IRB Issue Discussion among Faculty Members. A local colleague insisted that WKU has the IRB process but that is not a fact.
2023. 04.10 Checking the Chinese IRB Office Operation and Sent an Inquiry Email to the NHC (chinahealthgov@163.com) but failed.
2023. 04.08 (Working on Saturday for ORSP intentionally sent this request in late Friday to kill three working days for recipients): Dr. Choi raised 7 concerns and sugggestions that needs to be addressed before reviweing the WKU IRB Office Establishment.
2023. 04.07 (ORSP intentionally sent this request in late Friday to kill three working days for recipients): WKU ORSP sent an urgent review of four policy documents for WKU IRB's service with unclear scope of the purpose or sufficient information. The office request for the review by three workind days (April 12, 2023).
2023.04.03: Dr. Jerry Choi's Certificate of IRB Training Session: Human Subject Research: Humanities - Social Science - Education Research :
2023.04.01: Dr. Jerry Choi's Certificate of IRB Training Session: Human Subject Research: Social Behavioral Educational Research :
2023. 02.23: IRB applications Review could not be performed within WKU because WKU does not have a "Legitimated IRB Board" from Chinese Authorities. Either, there was no "Document" for IRB Board composition approval.
2022. 12.01: Completion of IRB Member Training Human Subject Research - IRB Member - Social Behavioral - Educational Focus (Curriculum, Course Learner) courses.
2022. 12. 28: IRB Online Training Program's Test
2022. 11. 14: Urgent request for "Completing CITI: IRB Members-Social-Behavioral-Educational Focus" Training course from WKU ORSP.
2021. 10. 28: Regulation of WKU Ethics Committee, Clarification - NOT FOR "Social Science & Education" Research.
2021. Mar. 29: Suggesting The Hybrid Model of IRB for WKU
2021. Mar. 24: Jin Chun (WKU ORSP) clarified the WKU needs to follow Kean USA IRB for research.
Mar. 24, 2021 WKU get the official approval of IRB establishment
2017.11.17: Initially Archived by Dr. Jerry Choi
'AmericanBizEdu@China > 1. Guides & Policy' 카테고리의 다른 글
| Guide for Advisement for Course Registration (WKU CBPM, MGM) (0) | 2018.11.22 |
|---|---|
| Kean Business Plan Competition (0) | 2018.01.23 |
| Guiding to Career Development for the Millenial. (0) | 2017.11.04 |
| Guiding to Graduate Schools Workshop (0) | 2017.10.11 |
| How to get a strong recommendation letter for Graduate school application? (0) | 2017.10.06 |









댓글